A New Era in
Gut Microbiome Science
is Here
The Mysteries of
the Human Gut Unveiled
Introducing CapScan —
The world’s first multi-region intestinal sampling device
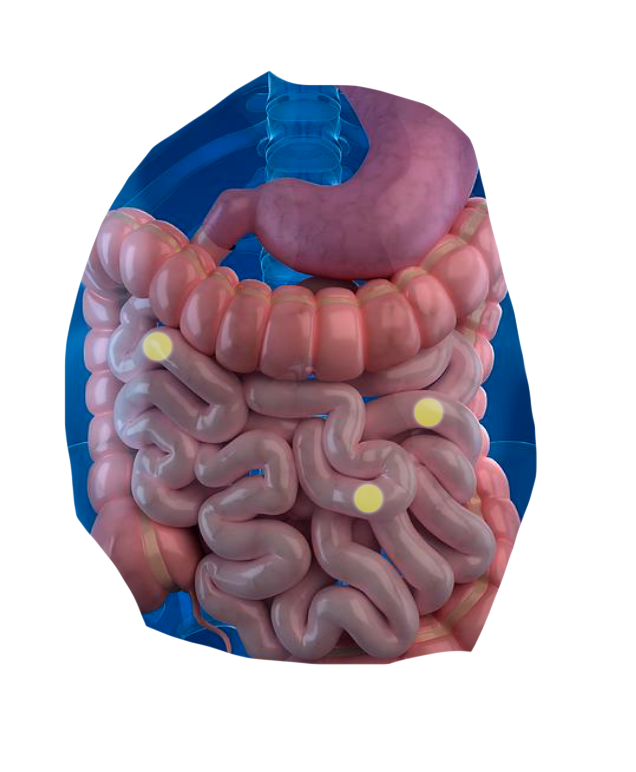
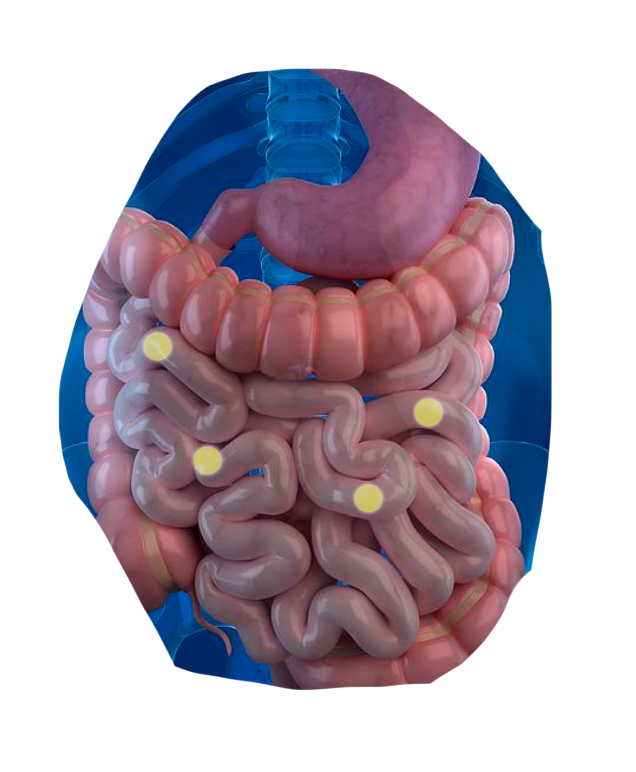
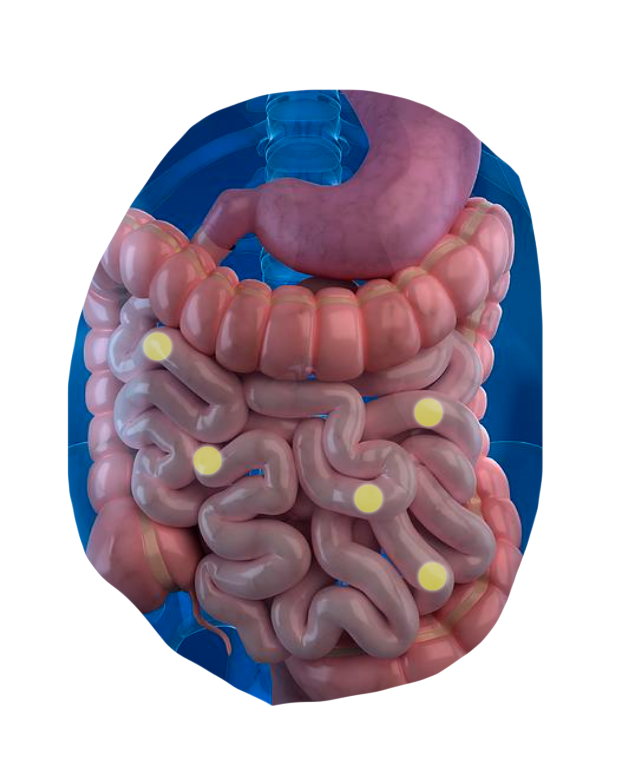
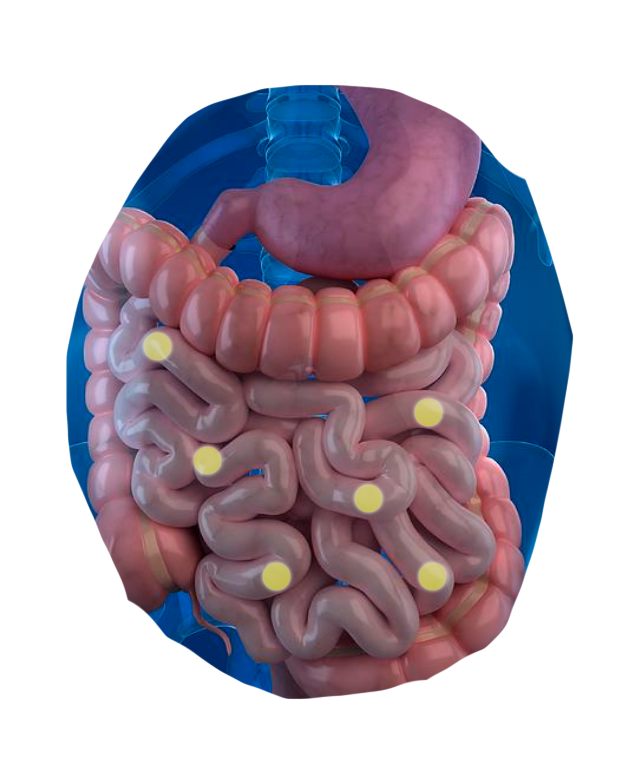
The CapScan® intestinal sampling device sheds light on vital gut activities that were previously unmeasurable.*

Pancreatic and liver secretions
CapScan enables the regional measurement of:
Small intestine microbiota
Secondary metabolites
Drug metabolism
Inflammatory biomarkers
Bile acid transformations
Fiber fermentations
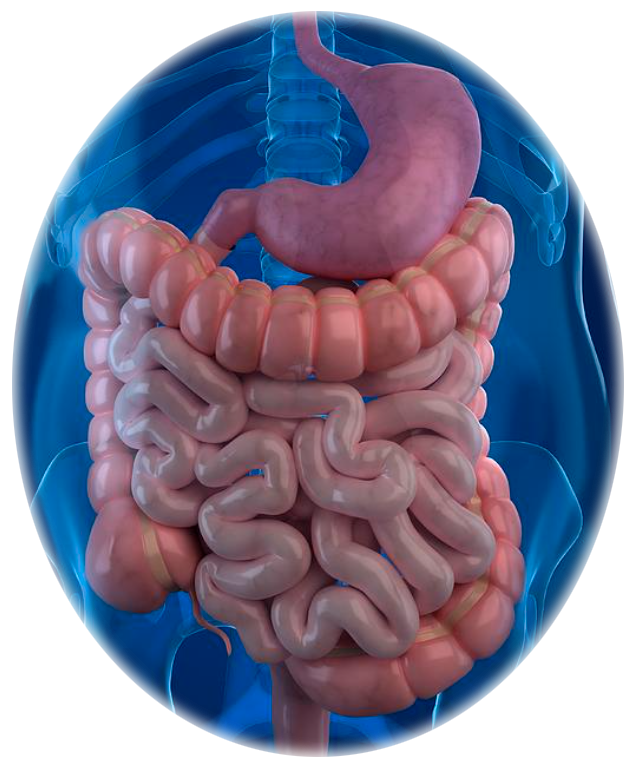
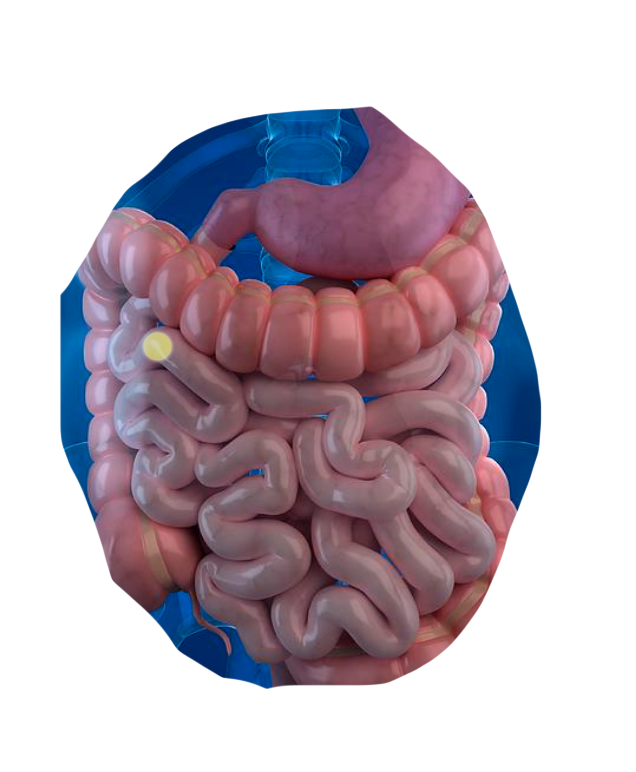
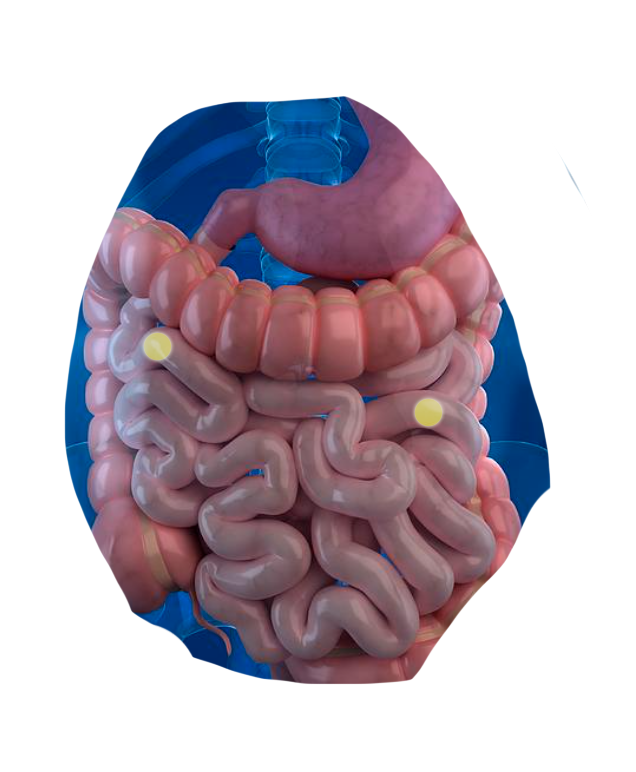
Our Science
We're leveraging CapScan and a multi-omics approach to discover novel host-microbiome interactions in the small intestine and ascending colon—
regions where key aspects of our metabolism, immunology, and neurology are regulated.
By directly sampling the human gut, we're bridging the gap between traditional oral and stool microbiome studies.
Our Science
CapScan is leading to science-driven discoveries in nutrition, digestive health and the gut microbiome.
Learn more about our cutting-edge research

Profiling the human intestinal environment under physiological conditions
Human metabolome variation along the upper intestinal tract
Watch the human gut and CapScan in action
The human gut is a dynamic physical and biochemical environment, where important biological activities occur at each of its different locations.
Using CapScan, we're non-invasively collecting samples throughout this complex environment.
Partnerships
Envivo’s unique combination of non-invasive intestinal sampling and cutting-edge analytics makes us the optimal partner to transform your microbiome research into new approaches for understanding and treating human disease.
Envivo is actively expanding its portfolio of partnerships with biotechnology firms, pharmaceutical companies, and academic scientists seeking to accelerate their clinical research and drug discovery and development programs.
If you would like to explore specific partnership opportunities, please contact us.
Funding Partners and Research Collaborations









About Us

Dari Shalon, PhD
Founder and CEO
Inventor of CapScan
PhD Stanford | Engineering & MBA MIT
Co-inventor of the DNA microarray &
Founder of Synteni
Founding Director Harvard Center for Genomic Research

Bennett Kapili, PhD
Founding Scientist
Microbial ecologist
PhD Stanford | BS Cornell
NSF Graduate Research Fellow
EDGE Fellow, Stanford
Academic Collaborators

David Relman, MD
Professor in Medicine, Microbiology & Immunology
Stanford University School of Medicine

Oliver Fiehn, PhD
Professor in the Department of Molecular and Cellular Biology
Director of Metabolomics
University of California Davis

KC Huang, PhD
Professor of Bioengineering, Microbiology & Immunology
Stanford University

Matthias Mann, PhD
Professor of Proteomics & Signal Transduction
Max Planck Institute of Biochemistry

George Triadafilopoulos, MD
Professor of Medicine
Department of Gastroenterology, Hepatology & Nutrition
University of Texas, MD Anderson Cancer Center

Andrew Patterson, PhD
Professor of Molecular Toxicology
Scientific Director of Metabolomics
Pennsylvania State University
News
New Nature Study Shows CapScan® Provides Novel Insights into the Human Gut Microbiome and Metabolome
 10 May 2023, San Francisco CA – Envivo Bio, a biotech company advancing the gut microbiome and metabolome field, announced that new data published in Nature show the company’s proprietary CapScan® intestinal sampling device can non-invasively and accurately profile the human gut microbiome and metabolome under physiological conditions for the first time. Findings from the landmark study suggest that CapScan, the first and only device of its kind, has the potential to accelerate microbiome-related research and biopharmaceutical drug-discovery and development programs.
10 May 2023, San Francisco CA – Envivo Bio, a biotech company advancing the gut microbiome and metabolome field, announced that new data published in Nature show the company’s proprietary CapScan® intestinal sampling device can non-invasively and accurately profile the human gut microbiome and metabolome under physiological conditions for the first time. Findings from the landmark study suggest that CapScan, the first and only device of its kind, has the potential to accelerate microbiome-related research and biopharmaceutical drug-discovery and development programs.Increasingly, research shows that the gut microbiome and metabolome play a critical role in numerous diseases as well as in food digestion, immune system regulation, and protection against pathogens, all of which have implications for human health and disease. Because the intestinal tract is regionally heterogenous, traditional sampling methods such as endoscopic biopsies and stool collection offer limited utility in understanding how intestinal gut microbes and metabolites impact human physiology.
In the Nature article, titled “Profiling the human intestinal environment under physiological conditions,” researchers from multiple universities and institutions describe CapScan’s capabilities to measure microbial, viral, proteomic, and bile acid profiles within the human intestines during normal digestion. The researchers report significant differences in microbiome composition, gene-class abundance, prophage induction, and the host proteome along various regions of the human intestine. Additionally, their findings detect and measure microbes and metabolites that are not present in stool or are inaccessible via endoscopic sampling.
“Our research confirms that, up until now, studies of the gut microbiome have really been studies of the stool microbiome, which missed out on most of the biological activity in our intestinal tract,” said Dari Shalon, Ph.D., founder and CEO of Envivo Bio. “By enabling researchers to sample and assess each of the diverse intestinal ecosystems separately and directly for the first time, CapScan opens the door to a new era of microbiome research.”
CapScan is a non-invasive, ingestible collection device that is about the size of a vitamin pill. Each device has a pH-targeted enteric coating, designed to dissolve at a pre-set rate based on the distinct pH levels of the various regions of the human intestines. Once this coating dissolves, CapScan’s internal bladder opens and draws in luminal content, which is then analyzed outside the body.
As detailed in the Nature paper, Shalon and collaborators from the Chan Zuckerberg Biohub, Max Planck Institute, Pennsylvania State University, Stanford University, University of California Davis, and two health care systems utilized CapScan to collect 240 intestinal samples from 15 healthy individuals. Each study participant ingested sets of four devices, which were all designed to open at progressively higher pH levels. Once the devices were evacuated, the scientists used multi-omics to analyze the massive sets of microbiome, metabolome, and proteome data collected regionally throughout the gastrointestinal tract.
“No one would expect to understand the headwaters of a river by sampling the river delta 1,000 miles downstream, yet this is how we’ve historically approached gut-related research,” said David A. Relman, M.D., Thomas C. and Joan M. Merigan professor of Medicine and professor of Microbiology & Immunology, Stanford School of Medicine, who co-led the study. “Our study demonstrates that there are differences between, as an example, bile acids in the small intestine versus stool samples that could be both scientifically and clinically meaningful.”
A companion study published in Nature Metabolism today describes additional spatiotemporal profiling of the gut metabolome using CapScan, including differences in dietary and lipid compounds. Taken together, the findings from both studies demonstrate the feasibility and utility of using CapScan to collect, characterize, and quantify the intestinal microbiota, metabolome, host proteins, and bile acids along the human intestine in a safe and routine manner.
“Our inability to see and measure the complex ecosystem of the gut has hindered our understanding of the features that make human intestines healthy or diseased, as well as our ability to develop new approaches to treat disease,” said Kerwyn Casey (KC) Huang, Ph.D., professor of Bioengineering and of Microbiology and Immunology, Stanford University, who co-led the study and is senior author on the Nature manuscript. “Our research suggests that we may be able to engineer new approaches to this problem.”
Envivo is also collaborating with researchers at Stanford Medicine on a clinical study using CapScan to understand the impact of the gut microbiome on the gut health of mothers and children in low- and middle-income countries with funding provided by the Bill & Melinda Gates Foundation.
“As we advance our CapScan technology with improved multi-regional sampling capabilities, we look forward to collaborating with biopharma companies and academic institutions to help improve our understanding of the highly complex gut microbiome and its role in health and disease,” added Dr. Shalon.
# # #
Contact:Michele Parisi
Bioscribe, Inc.
925-864-5028d
mparisi@bioscribe.com
New Nature Metabolism Study Demonstrates CapScan® Uniquely Profiles Dietary and Lipid Compounds in the Gut Metabolome
 10 May 2023, San Francisco CA – Envivo Bio, a biotech company advancing the gut microbiome and metabolome field, announced that new data published in Nature Metabolism show the company’s proprietary CapScan® intestinal sampling device can provide spatial and temporal insights into the human metabolome within the upper intestinal tract. This is the second publication demonstrating CapScan’s ability to uncover the multi-regional, dynamic data needed to provide an accurate, comprehensive picture of gut physiology.
10 May 2023, San Francisco CA – Envivo Bio, a biotech company advancing the gut microbiome and metabolome field, announced that new data published in Nature Metabolism show the company’s proprietary CapScan® intestinal sampling device can provide spatial and temporal insights into the human metabolome within the upper intestinal tract. This is the second publication demonstrating CapScan’s ability to uncover the multi-regional, dynamic data needed to provide an accurate, comprehensive picture of gut physiology.CapScan is a non-invasive, ingestible collection device that is the size of a standard vitamin pill. Each device has a pH-targeted enteric coating, designed to dissolve at a pre-set rate based on the distinct pH levels of the various regions of the human intestines. Once this coating dissolves, CapScan’s internal bladder opens and draws in luminal content, which is then analyzed outside the body. CapScan’s multi-regional sampling capabilities allow the spatiotemporal measurement of pancreatic and liver secretions, small intestine microbiota, secondary metabolites, drug metabolism, inflammatory biomarkers, bile acid transformations, and fiber fermentations.
In the Nature Metabolism article, titled “Human metabolome variation along the upper intestinal tract,” researchers from University of California, Davis evaluated CapScan’s ability to study the spatiotemporal variation of upper intestinal metabolome during routine daily digestion in 15 healthy subjects. The scientists identified approximately 1,900 metabolites, including sulfonolipids and fatty acid esters of hydroxy fatty acids (FAHFA) lipids, some of which had never before been detected in human samples. They also identified associations between metabolites and dietary biomarkers, and luminal keto acids and fruit intake.
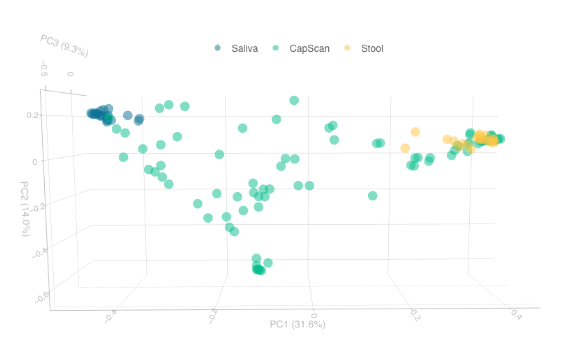
Importantly, results revealed that the stool metabolome differed significantly from the intestinal metabolome, suggesting that analyzing stool does not provide an accurate representation of the metabolites present in the intestines. As an example, 31 metabolites were >100 times more abundant on average in the intestine comparted with stool. In addition, researchers found significant variability of the metabolome along the intestinal tract itself, highlighting the regional and dynamic nature of the gut.
“Researchers have been limited in evaluating gut metabolites due to the lack of sampling capability along the entire intestinal tract,” said Dari Shalon, Ph.D., founder and CEO of Envivo Bio. “Together, our Nature Metabolism publication and our companion landmark Nature article show that CapScan can provide extraordinary insights into the inner microbial and metabolic mechanisms of gut activities beyond what scientists have been able to study in stool or with endoscopic sampling.”
In the companion Nature article, titled “Profiling the human intestinal environment under physiological conditions,” researchers from multiple universities and institutions describe CapScan’s ability to measure microbial, viral, proteomic, and bile acid profiles within the human intestines during normal digestion.
“Taken together, these two studies illustrate the utility and importance of sampling directly from the intestines to enhance our understanding of the relationship between us, our commensal microbes and the gut metabolome,” said Oliver Fiehn, Ph.D., professor of Molecular and Cellular Biology at the University of California, Davis, and senior author on the Nature Metabolism paper. “Routine access to intestinal samples will advance research into human nutrition, and could potentially lead to new therapies to human disease.”
# # #
Contact:Michele Parisi
Bioscribe, Inc.
925-864-5028d
mparisi@bioscribe.com
National Science Foundation Awards Envivo Bio a Phase 1 SBIR Grant
 22 January 2020, San Carlos CA – The National Science Foundation (NSF) has awarded a Phase 1 Small Business Innovation Research (SBIR) grant to Envivo Bio to support the development of CapScan®, a groundbreaking innovation aimed at non-invasive sampling of the human gut microbiota and metabolites.
22 January 2020, San Carlos CA – The National Science Foundation (NSF) has awarded a Phase 1 Small Business Innovation Research (SBIR) grant to Envivo Bio to support the development of CapScan®, a groundbreaking innovation aimed at non-invasive sampling of the human gut microbiota and metabolites.The NSF SBIR program is designed to encourage small businesses to develop innovative solutions that have the potential to make significant societal and economic impacts. Envivo Bio is honored to have been selected for this grant, which will enable the company to accelerate the development of CapScan and bring it to market.
CapScan is an ingestible gut sampling devices that promises to advance human gut microbiome research. The grant will enable Envivo Bio to further develop the technology and conduct necessary research and development, including testing and prototyping, to move the project towards commercialization.
"We are thrilled to receive this grant from the National Science Foundation," said Dari Shalon, PhD, founder and CEO of Envivo Bio and the inventor of CapScan. "This award will allow us to continue our work on CapScan intestinal sampling device, which has the potential to shed new light on the human gut microbiota. We look forward to working with the NSF and other partners to bring this innovation to the market and make a meaningful impact on society."
Envivo Bio Awarded NSF SBIR Phase 2 Grant for Innovative Technology Development
 10 September 2021, San Carlos CA – Envivo Bio has been awarded a National Science Foundation (NSF) Small Business Innovation Research (SBIR) Phase 2 grant to continue the development of the CapScan® ingestible gut sampling device. The NSF SBIR program is highly competitive and supports small businesses with innovative and marketable ideas that have the potential to make a significant impact in a scientific field.
10 September 2021, San Carlos CA – Envivo Bio has been awarded a National Science Foundation (NSF) Small Business Innovation Research (SBIR) Phase 2 grant to continue the development of the CapScan® ingestible gut sampling device. The NSF SBIR program is highly competitive and supports small businesses with innovative and marketable ideas that have the potential to make a significant impact in a scientific field.The Phase 2 grant is the second round of funding provided by the NSF SBIR program to Envivo, designed to support the continued development and commercialization of this promising technology. Envivo Bio was previously awarded a Phase 1 grant to conduct feasibility studies and develop a proof-of-concept of the CapScan device.
"We are honored to have been selected for a Phase 2 grant from the NSF SBIR program," said Dari Shalon PhD, CEO of Envivo Bio. "This funding will allow us to continue the development of our innovative technology, which has the potential to revolutionize the gut microbiome field. We are grateful for the support of the NSF and look forward to introducing our technology to the market."
Envivo Bio's technology is expected to have significant impact on the gut microbiome field by enabling the sampling of the small intestines in a safe and non-invasive manner. With the Phase 2 grant, Envivo Bio will be able to further refine and optimize the CapScan technology, conduct more extensive testing, and move closer to commercialization.
The NSF SBIR program provides critical funding and support to small businesses across the country, helping to spur innovation and promote economic growth. The Phase 2 grant awarded to Envivo Bio is a testament to the potential of its technology and the strength of its business plan.
NIH Awards Grant for Development and Clinical Evaluation of the CapScan® Device
 24 March 2022, San Carlos CA – Envivo Bio has been awarded a grant from the National Cancer Institute (NCI) of the National Institutes of Health (NIH) to support the development and clinical evaluation of CapScan®. The CapScan Device is a non-invasive device that samples the gut microbiome and metabolome in a safe and non-invasive manner.
24 March 2022, San Carlos CA – Envivo Bio has been awarded a grant from the National Cancer Institute (NCI) of the National Institutes of Health (NIH) to support the development and clinical evaluation of CapScan®. The CapScan Device is a non-invasive device that samples the gut microbiome and metabolome in a safe and non-invasive manner.The NIH grant will support the continued development of the CapScan device, including clinical trials to evaluate its efficacy and usability. The goal of the project is to demonstrate that the CapScan device is a reliable tool that sheds new light on the activities of the gut microbes and their associated metabolites in health and disease.
"We are thrilled to receive this grant from the NIH, which will allow us, together with our collaborators at Stanford University and the University of California Davis, to continue developing the CapScan device and demonstrate its potential to improve our understanding of the gut microbiome," said Dari Shalon PhD, Founder and CEO of Envivo Bio. "CapScan is a novel device that explores the under-explored world of small intestine physiology. We believe that CapScan has the potential to provide a more accurate picture of most gut activities as compared to current methods."
The CapScan device has the potential to revolutionize the way scientists and clinicians measure the microbial and biochemical activities in the small intestines. With the support of the NIH grant, Envivo Bio is excited to continue developing this innovative technology and bring it to market in the near future.
Envivo Bio to Study Environmental Enteropathy Using its CapScan® Intestinal Sampling Device
 22 May 2022, San Carlos, CA – Envivo Bio will participate in an environmental enteropathy study, led by researchers from Stanford Medicine, that uses its novel CapScan intestinal sampling device. The study is funded by a grant from the Bill & Melinda Gates Foundation. The study aims to understand the impact of the gut microbiome on the gut health of mothers and children in low and middle income countries.
Environmental Enteric Dysfunction (EED) is a chronic condition that affects gut health, leading to malnutrition, stunted growth, and other health problems. The condition is prevalent in many parts of the world, particularly in sub-Saharan Africa and South Asia, where access to clean water and sanitation facilities is limited.
The CapScan is a non-invasive device that samples the microbes and metabolites in the small intestines. The research team will use the CapScan device to analyze the gut microbiome of pregnant women in Bangladesh, Zambia, Pakistan and Senegal.
"We are thrilled to be working with David Relman, MD, and his Stanford Medicine team to use this cutting-edge technology to study environmental enteropathy in a way that has never been done before," said Dari Shalon PhD, Founder and CEO of Envivo Bio. "By understanding the impact of the gut microbiota and associated metabolites on the gut health of expecting mothers, we may be able to facilitate development of targeted interventions to improve the health and well-being of these women and their babies."
David Relman, MD, is the Thomas C. and Joan M. Merigan Professor of Medicine, and Professor of Microbiology & Immunology at Stanford Medicine.
The Envivo and Stanford Medicine research team will work closely with in-country experts to collect data and samples from pregnant women. These data will be analyzed using advanced bioinformatics tools to identify key microbial and metabolites markers that can be used to diagnose and assess the effectiveness of future treatments for EED.
The Envivo team hopes that the study will inform the development of new diagnostic tools and interventions to address EED and improve the health and well-being of women and children in some of the world's most vulnerable communities.
22 May 2022, San Carlos, CA – Envivo Bio will participate in an environmental enteropathy study, led by researchers from Stanford Medicine, that uses its novel CapScan intestinal sampling device. The study is funded by a grant from the Bill & Melinda Gates Foundation. The study aims to understand the impact of the gut microbiome on the gut health of mothers and children in low and middle income countries.
Environmental Enteric Dysfunction (EED) is a chronic condition that affects gut health, leading to malnutrition, stunted growth, and other health problems. The condition is prevalent in many parts of the world, particularly in sub-Saharan Africa and South Asia, where access to clean water and sanitation facilities is limited.
The CapScan is a non-invasive device that samples the microbes and metabolites in the small intestines. The research team will use the CapScan device to analyze the gut microbiome of pregnant women in Bangladesh, Zambia, Pakistan and Senegal.
"We are thrilled to be working with David Relman, MD, and his Stanford Medicine team to use this cutting-edge technology to study environmental enteropathy in a way that has never been done before," said Dari Shalon PhD, Founder and CEO of Envivo Bio. "By understanding the impact of the gut microbiota and associated metabolites on the gut health of expecting mothers, we may be able to facilitate development of targeted interventions to improve the health and well-being of these women and their babies."
David Relman, MD, is the Thomas C. and Joan M. Merigan Professor of Medicine, and Professor of Microbiology & Immunology at Stanford Medicine.
The Envivo and Stanford Medicine research team will work closely with in-country experts to collect data and samples from pregnant women. These data will be analyzed using advanced bioinformatics tools to identify key microbial and metabolites markers that can be used to diagnose and assess the effectiveness of future treatments for EED.
The Envivo team hopes that the study will inform the development of new diagnostic tools and interventions to address EED and improve the health and well-being of women and children in some of the world's most vulnerable communities.Envivo Bio Awarded NSF SBIR TECP Grant for Innovative Medical Device
 9 December 2022, San Carlos CA – Envivo Bio, a leader in gut microbiome research, has been awarded a National Science Foundation (NSF) Small Business Innovation Research (SBIR) Technology Enhancement for Commercial Partnerships (TECP) grant to support the development of its novel CapScan® intestinal sampling device.
9 December 2022, San Carlos CA – Envivo Bio, a leader in gut microbiome research, has been awarded a National Science Foundation (NSF) Small Business Innovation Research (SBIR) Technology Enhancement for Commercial Partnerships (TECP) grant to support the development of its novel CapScan® intestinal sampling device.The grant will fund the research and development of this cutting-edge device that will help researchers explore the activity of the trillions of microbes and hundreds of thousands of metabolites in the human intestinal tract. The device will be a breakthrough in gut microbiome research, providing a non-invasive, safe and effective tool to access a part of the human anatomy that has evaded systematic study to date.
"We are thrilled to have been awarded this grant, which recognizes the innovative potential of our technology," said Dari Shalon PhD, Founder and CEO at Envivo Bio. "This grant will enable us to continue our research and development efforts, bringing us one step closer bringing CapScan to the market."
The SBIR TECP grant is a highly competitive funding opportunity that supports small businesses that are developing innovative technologies with high commercial potential. The grant provides funding to help businesses partner with industry leaders to accelerate the commercialization of their products.
"The NSF SBIR TECP grant is a significant achievement for our team and demonstrates our commitment to developing this innovative medical technology," said Dr. Shalon. "We look forward to working with our industry partners to bring this device to market and eventually improve patient care."
Envivo Bio is dedicated to uncovering the mysteries of the human gut microbiome and developing novel therapeutic approaches based on these insights. The company’s management has a strong track record of success in developing novel medical devices and is committed to advancing the gut microbiome field for the benefit of researchers, clinicians and patients worldwide.
Contact Us
*CapScan is an Investigational Device. Limited by federal law to investigational use only.